新生血管/血管生成
![]()
PSI 用于新生血管/血管生成
新生血管是新的微血管网络的形成。在体内,这一过程主要通过血管生成实现,即新血管从现有血管中生长出来。新生血管/血管生成在许多治疗和病理中均扮演至关重要的角色,这也是其在许多领域被广泛研究的原因,其中包括组织工程和再生医学、血管生物学和癌症生物学。根据具体应用,存在许多用于药物开发/配送、疾病表征和干细胞治疗的模型。我们需要能够用于评估新血管形成的工具,最好通过非侵入性和非破坏性的方法实现。PeriCam PSI 激光散斑对比成像系统可用于跟踪微血管血流灌注随时间的变化。研究表明,灌注的增加与功能更强的血管形成相对应。PeriCam PSI 已推出多种型号产品。
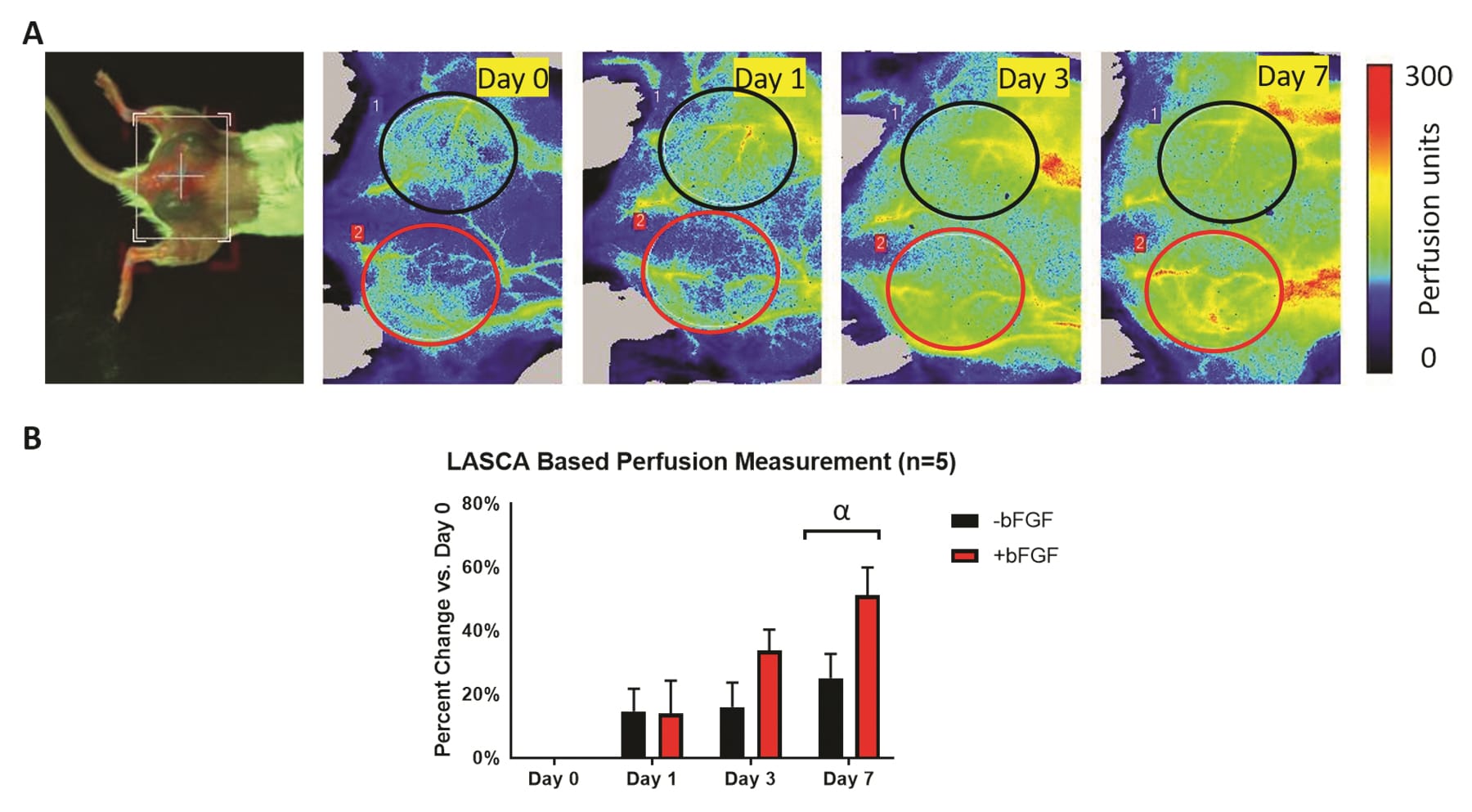
(A)植入有两种皮下纤维蛋白支架植入物(分别有/无血管生长因子)的小鼠的背视图照片和纵向 PSI 灌注图像。根据植入物的物理位置选择 ROI。(B)相对于第0天的灌注变化的定量(基于对 PSI 灌注图像的 ROI 分析)显示灌注随时间总体增加。灌注的最大变化在第 7 天观察到,有 bFGF 支架比无 bFGF支架 1-2 有更大的灌注趋势。
图片由 Mario Fabiilli 博士提供,来自基于 LED 的光声成像用于监测纤维蛋白支架的血管生成,作者:Yunhao Zhu、Xiaofang Lu、Xiaoxiao Dong等,出版物:组织工程学 C 部分:方法
出版商:Mary Ann Liebert,Inc.日期:2019 年 9 月 1 日。
概述
PeriCam PSI 已被证明是新生血管和血管生成研究领域中一系列不同分领域的有用工具。一个主要的分领域是在临床前动物模型中研究促血管生成疗法治疗外周动脉疾病 (PAD) 和伤口愈合。针对 PAD,通过手术在下肢诱导缺血,并使用 PeriCam PSI 进行确认,随着时间的推移,可以跟踪灌注恢复的动态过程。以同样的方式造成皮肤伤口,随后使用 PeriCam PSI 跟踪正常愈合过程。在两种模型中,PeriCam PSI 均能够使研究人员通过减少研究所需的动物数量,纵向跟踪相同的受试动物,从而节省时间和金钱。
另一个分领域是在 CAM 检测和皮下肿瘤模型中进行癌症研究以及治疗用抗血管生成药物的开发。PeriCam PSI 可用于监测肿瘤进展过程中的灌注情况,并评估旨在对其进行饥饿治疗的疗法的疗效。
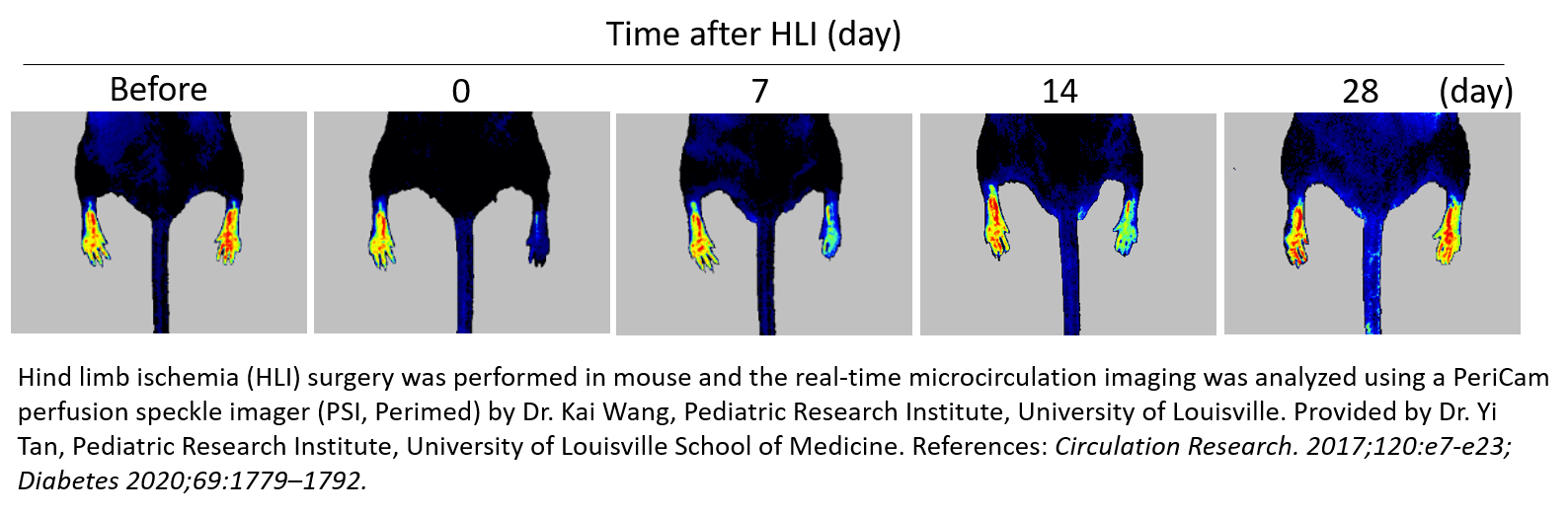
后肢缺血 (HLI)
“小鼠后肢缺血”方法是研究外周动脉疾病的常用和重要模型。它涉及单侧股动脉结扎和切除以诱导急性肢体缺血,从而通过多种策略(如生长因子递送和细胞治疗)进行血管再生、血管生成和动脉生成的评估。它也可用于评估治疗药物 3-12 的促血管生成作用,或研究健康人群 13 与患病(糖尿病)人群14-16 、年轻人与老年人人群 17 以及野生型与基因敲除动物 18-20 中的自然恢复。
通过测量微循环中血流灌注的减少 (60-70%),PeriCam PSI 激光散斑对比成像系统可用于确认手术后缺血并纵向监测治疗策略的疗效。通常,在使用 PSI 进行手术/治疗后,对每只动物进行长达一个月的跟踪,以记录血管重建后血流灌注的逐步增加,从而通过减少研究所需的动物数量,节省研究人员的时间和成本。
鸡胚绒毛尿囊膜 (CAM) 试验
在鸡胚发育过程中,CAM 是由尿囊体的中胚层与绒毛膜的中胚层融合形成的。它是一种高度血管化、非神经支配的胚胎外膜,是研究血管生成和肿瘤生长的理想底物。将受精卵孵育 4 至 8 天,同时在壳中开设窗口以观察形成的胚胎和血管时。
对于血管生成试验,各种生物分子和药物可以进行局部输送并研究其血管生成效力。也可植入生物材料支架并监测血管侵袭随时间的变化,以评估组织工程学策略。
对于癌症模型,可以将各种类型的癌症细胞移植到 CAM 中以进行肿瘤培育。这为研究许多不同的癌症肿瘤形成提供了一个相对简单的模型,从而使我们可以测试新治疗药物和个性化治疗策略。CAM 模型具有高度的可重复性和成本效益,并且不像许多其他活体试验动物模型那样需要伦理委员会的批准。此外,CAM 模型具有自然免疫缺陷,非常适合细胞移植,而且,封闭的系统延长了许多实验分子的半衰期,减少了研究所需数量。
PeriCam PSI 激光散斑对比成像系统可用于在血管生成试验中测量血流灌注的变化,并检测各种促血管生成化合物的功效差异。它还可用于监测功能性肿瘤内血管 25 的形成,并评估用于癌症治疗的抗血管生成剂的有效性。
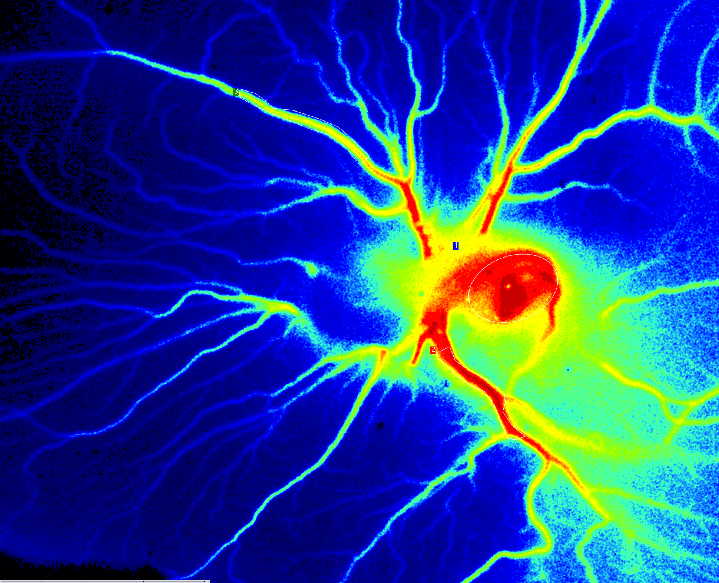
受精后第 6 天 PSI Z 灌注 CAM 检测。
皮下肿瘤
肿瘤的生长和转移取决于血管的发育和有效的微循环。如果没有血管生成,小的肿瘤细胞病灶就不能在继发部位生长。由于血管生成在肿瘤生长、进展、侵袭和转移中起到核心作用,对其进行抑制可为一些癌症治疗方式提供潜在的策略。
皮下肿瘤模型是新型抗癌候选药物体内评价最受欢迎的评估系统。通常使用免疫缺陷动物品系,将培养的癌细胞皮下植入,在约2周内会导致实体瘤形成。已对多种人和鼠癌细胞系进行了调整,使其能够在啮齿类动物宿主中生长,从而允许在相关肿瘤模型中对治疗药物进行评价。研究人员监测肿瘤生长和进展,并经常进行肿瘤微血管密度测定,以评估其治疗策略的疗效。PeriCam PSI 可用于监测皮下肿瘤随时间的血流灌注,并评估抗血管生成疗法治疗癌症的疗效。
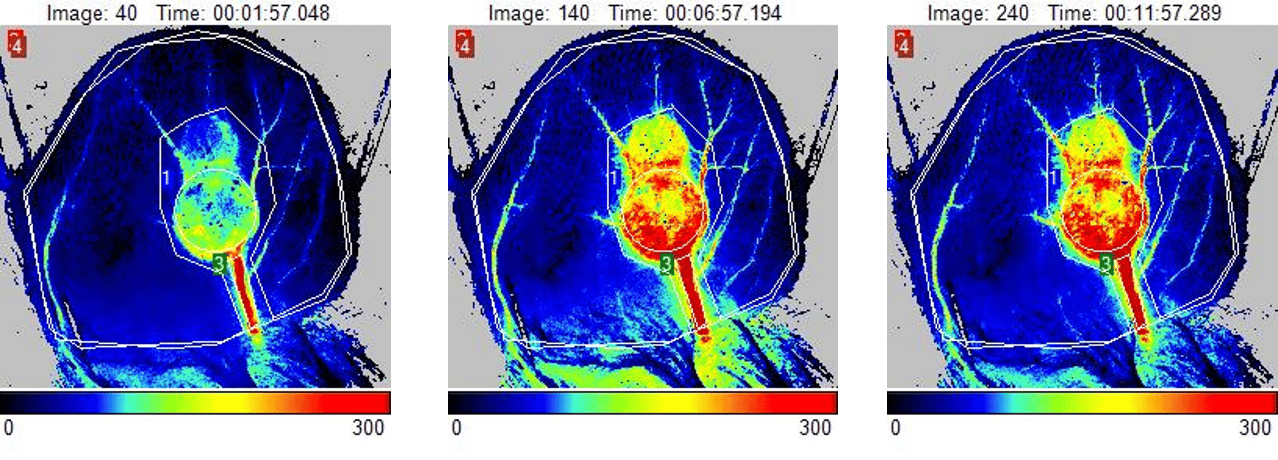
第 14 天皮下肿瘤小鼠耳部的灌注图像。图像反映了加热挑战引起的肿瘤血流灌注增加。
References:
1. LED-Based Photoacoustic Imaging for Monitoring Angiogenesis in Fibrin Scaffolds. Yunhao Zhu, Xiaofang Lu, Xiaoxiao Dong, Jie Yuan, Mario L. Fabiilli, and Xueding Wang. 9, September 2019, Tissue Engineering Part C: Methods, Vol. 25, pp. 523-531.
2. Controlled release of basic fibroblast growth factor for angiogenesis using acoustically-responsive scaffolds. Alexander Moncion, Melissa Lin, Eric G. O’Neill, Renny T. Franceschi, Oliver D. Kripfgans, Andrew J. Putnam, Mario L. Fabiilli. 2017, Biomaterials, Vol. 140, pp. 26-36.
3. MiR-15b-5p Regulates Collateral Artery Formation by Targeting AKT3 (Protein Kinase B-3). Ling-Ping Zhu, Ji-Peng Zhou, Jia-Xiong Zhang, Jun-Yao Wang, Zhen-Yu Wang, Miao Pan, Ling-. May 2017, Arteriosclerosis, Thrombosis, and Vascular Biology, Vol. 37, pp. 957-968.
4. Precise and long-term tracking of adipose-derived stem cells and their regenerative capacity via superb bright and stable organic nanodots. Dan Ding, Duo Mao, Kai Li, Xiaomin Wang, Wei Qin, Rongrong Liu, David Shunzhong Chiam, Nikodem Tomczak, Zhimou Yang, Ben Zhong Tang, Deling Kong, Bin Liu. 12, 2014, ACS Nano, Vol. 8, pp. 12620-12631.
5. Extracellular Matrix Hydrogel Promotes Tissue Remodeling, Arteriogenesis, and Perfusion in a Rat Hindlimb Ischemia Model. Jessica L. Ungerleider, Todd D. Johnson, Melissa J. Hernandez, Dean I. Elhag, Rebecca L. Braden, Monika Dzieciatkowska, Kent G. Osborn, Kirk C. Hansen, Ehtisham Mahmud and Karen L. Christman. 1-2, 2016, JACC: Basic to Translational Science, Vol. 1, pp. 32-44.
6. Engineering an Injectable Muscle-Specific Microenvironment for Improved Cell Delivery Using a Nanofibrous Extracellular Matrix Hydrogel. Nikhil Rao, Gillie Agmon, Matthew T. Tierney, Jessica L. Ungerleider, Rebecca L. Braden, Alessandra Sacco, Karen L. Christman. 2017, ACS Nano, Vol. 11, pp. 3851-3859.
7. Marrow-isolated adult multilineage inducible cells embedded within a biologically-inspired construct promote recovery in a mouse model of peripheral vascular disease. Cristina Grau-Monge, Gaëtan J-R Delcroix, Andrea Bonnin-Marquez, Mike Valdes, Ead Lewis Mazen Awadallah, Daniel F Quevedo, Maxime R Armour, Ramon B Montero, Paul C Schiller, Fotios M Andreopoulos and Gianluca D’Ippolito. 1, 2017, Biomedical Materials, Vol. 12, p. 015024.
8. Natural compound bavachalcone promotes the differentiation of endothelial progenitor cells and neovascularization through the RORα-erythropoietin-AMPK axis. Shuang Ling, Rong-Zhen Ni, Yunyun Yuan, Yan-Qi Dang, Qian-Mei Zhou, Shuang Liang, Fujiang Guo, Wei Feng, Yuanyuan Chen, Katsumi Ikeda, Yukio Yamori, and Jin-Wen Xu. 49, 2017, Oncotarget, Vol. 8, pp. 86188-86205.
9. Topical tissue nano-transfection mediates non-viral stroma reprogramming and rescue. Daniel Gallego-Perez, Durba Pal, Subhadip Ghatak, VeysiMalkoc, Natalia Higuita-Castro, Surya Gnyawali, Lingqian Chang, Wei-Ching Liao, Junfeng Shi, Mithun Sinha, Kanhaiya Singh, Erin teen, Alec Sunyecz, Richard Stewart, JordanMoore, Thomas Ziebro, Robert. 2017, Nature Nanotechnology, Vol. 12, pp. 974-979.
10. In vivo efficacy of endothelial growth medium stimulated mesenchymal stem cells derived from patients with critical limb ischemia. Rida Al-Rifai, Philippe Nguyen, Nicole Bouland, Christine Terryn, Lukshe Kanagaratnam, Gaël Poitevin, Caroline François, Catherine Boisson-Vidal, Marie-Antoinette Sevestre & Claire Tournois. 17, 2019, Journal of Translational Medicine, Vol. 9, p. 261.
11. NFAT5 promotes arteriogenesis via MCP‐1‐dependent monocyte recruitment. Zhang, Xing‐Chi Lin Miao Pan Ling‐Ping Zhu Quan Sun Zheng‐Shi Zhou Chuan‐Chang Li Guo‐Gang. 2, 2020, Journal of Cellular and Molecular Medicine, Vol. 24.
12. MicroRNA-146a Regulates Perfusion Recovery in Response to Arterial Occlusion via Arteriogenesis. Heuslein Joshua L., McDonnell Stephanie P., Song Ji, Annex Brian H., Price Richard J. 2018, Frontiers in Bioengineering and Biotechnology, Vol. 6, p. 1.
13. Denervation in Femoral Artery-Ligated Hindlimbs Diminishes Ischemic Recovery Primarily via Impaired Arteriogenesis. Yinghuan Cen, Junfeng Liu, Yuansen Qin, Ruiming Liu, Huijin Wang, Yu Zhou, Shenming Wang, Zuojun Hu. 5, 2016, PLOS One, Vol. 11, p. e0154941.
14. Elevating CXCR7 Improves Angiogenic Function of EPCs via Akt/GSK-3β/Fyn-Mediated Nrf2 Activation in Diabetic Limb Ischemia. Xiaozhen Dai, Xiaoqing Yan, Jun Zeng, Jing Chen, Yuehui Wang, Jun Chen, Yan Li, Michelle T Barati, Kupper A Wintergerst, Kejian Pan, Matthew A Nystoriak, Daniel J Conklin, Gregg Rokosh, Paul N Epstein, Xiaokun Li, Yi Tan. 2017, Circulation Research, Vol. 120, pp. e7-e23.
15. Sitagliptin-mediated preservation of endothelial progenitor cell function via augmenting autophagy enhances ischaemic angiogenesis in diabetes. Dai, X., Zeng, J., Yan, X., Lin, Q., Wang, K., Chen, J., Shen, F., Gu, X., Wang, Y., Chen, J., Pan, K., Cai, L., Wintergerst, K. A. and Tan, Y. 1, 2018, Journal of Cellular and Molecular Medicine, Vol. 22, pp. 89-100.
16. Endothelial Overexpression of Metallothionein Prevents Diabetes-Induced Impairment in Ischemia Angiogenesis Through Preservation of HIF-1α/SDF-1/VEGF Signaling in Endothelial Progenitor Cells. Kai Wang, Xiaozhen Dai, Junhong He, Xiaoqing Yan, Chengkui Yang, Xia Fan, Shiyue Sun, Jing Chen, Jianxiang Xu, Zhongbin Deng, Jiawei Fan, Xiaohuan Yuan, Hairong Liu, Edward C. Carlson, Feixia Shen, Kupper A. Wintergerst, Daniel J. Conklin, Paul N. Epstein. 8, August 2020, Diabetes, Vol. 69, pp. 1779-1792.
17. DNA Methyltransferase 1–Dependent DNA Hypermethylation Constrains Arteriogenesis by Augmenting Shear Stress Set Point. Joshua L. Heuslein, Catherine M. Gorick, Ji Song, Richard J. Price. 12, 2017, Journal of the American Heart Association, Vol. 6.
18. Loss of Endothelial CXCR7 Impairs Vascular Homeostasis and Cardiac Remodeling After Myocardial Infarction. Huifeng Hao, PhD, et al. 2017, Circulation, Vol. 135, pp. 1253-1264.
19. Vascular growth responses to chronic arterial occlusion are unaffected by myeloid specific focal adhesion kinase (FAK) deletion. Joshua L. Heuslein, Kelsey P. Murrell, Ryan J. Leiphart, Ryan A. Llewellyn, Joshua K. Meisner & Richard J. Price. 2016, Scientific Reports, Vol. 6, p. 27029.
20. Despite Normal Arteriogenic and Angiogenic Responses, Hindlimb Perfusion Recovery and Necrotic and Fibro-Adipose Tissue Clearance Are Impaired in MMP9 Deficient Mice. Meisner JK, Annex BH, Price RJ. 6, 2015, Journal of Vascular Surgery, Vol. 61, pp. 1583–1594.
21. Cutaneous Epithelial to Mesenchymal Transition Activator ZEB1 Regulates Wound Angiogenesis and Closure in a Glycemic Status–Dependent Manner. Singh, Kanhaiya and Sinha, Mithun and Pal, Durba and Tabasum, Saba and Gnyawali, Surya C. and Khona, Dolly and Sarkar, Subendu and Mohanty, Sujit K. and Soto-Gonzalez, Fidel and Khanna, Savita and Roy, Sashwati and Sen, Chandan K. 11, 2019, Diabetes, Vol. 68, pp. 2175-2190.
22. Correction of MFG-E8 Resolves Inflammation and Promotes Cutaneous Wound Healing in Diabetes. A. Das, S. Ghatak, M. Sinha, S. Chaffee, Noha S. Ahmed, N. L. Parinandi, E. S. Wohleb, J. F. Sheridan, C. K. Sen, and S. Roy. 12, 2016, The Journal of Immunology, Vol. 196, pp. 5089-5100.
23. Circulating Exosomal miR-20b-5p Inhibition Restores Wnt9b Signaling and Reverses Diabetes-Associated Impaired Wound Healing. Yuan Xiong, Lang Chen, Chenchen Yan, Wu Zhou, Yori Endo, Jing Liu, Liangcong Hu,Yiqiang Hu, Bobin Mi and Guohui Liu. 3, 2020, Small, Vol. 16, p. 1904044.
24. Saliva Exosomes-Derived UBE2O Promotes Angiogenesis in Cutaneous Wounds by Targeting SMAD6. Bobin Mi, Lang Chen, Yuan Xiong, Chenchen Yan, Hang Xue, Adriana C. Panay, Jing Liu, Liangcong Hu, Yiqiang Hu, Yun Sun, Faqi Cao, Wu Zhou, Guohui Liu. 18, 2020, Journal of Nanobiotechnology, Vol. 6, p. 68.
25. Laser speckle contrast analysis (LASCA) technology for the semiquantitative measurement of angiogenesis in in-ovo-tumor-model. Eric Pion, Claudia Asam, Anna-Lena Feder, Oliver Felthaus, Paul I. Heidekrueger,Lukas Prantl, Silke Haerteis, Thiha Aung. 2020, Microvascular Research, Vol. 133, p. 104072.
请填写表单,以获取更多信息
免责声明:Perimed 网站上的产品并非在全球所有市场均获准销售。



