Case example:
The Microcirculation in Obesity and type 2 Diabetes
Dr. Alfons J.H.M. Houben Dept. of Internal Medicine, Maastricht University Medical Center+ and School for Cardiovascular Diseases (CARIM), Maastricht, The Netherlands
The focus of my research for many years has been microvascular dysfunction (MVD) as both a cause and a consequence of (cardio)metabolic diseases (see 1 for our working hypothesis). One important function of the microcirculation is to deliver oxygen/nutrients to all tissues and to remove waste products. In normal metabolism this includes the delivery of glucose, which taken up by the gut following a meal, to skeletal muscle in order to be stored as glycogen. For that, insulin plays an important role by stimulating the endothelium to produce nitric oxide (NO), which leads to recruitment of capillaries in skeletal muscle and thus an increase in exchange surface. As a result, both glucose and insulin can reach skeletal muscle cells quickly and easily in order to store glucose in the cells. In our working hypothesis we state that MVD may disturb this insulin-mediated glucose delivery/uptake, leading to metabolic insulin resistance and contributing to the development of type 2 diabetes (1).
Important determinants of (early) MVD are amongst others: (epi)genetics, low birth weight, physical inactivity, and aging. In addition, obesity has been shown to be an important determinant of MVD. Different pathways are disturbed in obesity (e.g. increased inflammation, increased oxidative stress, increased free fatty acids, decreased adiponectin etc.), all influencing normal signaling pathways in both endothelial and vascular smooth muscle cells (2).
Once metabolic insulin resistance or type 2 diabetes has developed, this will lead to higher levels and prolonged periods of hyperglycemia following meals. Because glucose is easily taken up by endothelial cells, these periods of hyperglycemia will further aggravate microvascular endothelial function. Intracellular hyperglycemia leads to the production of methylglyoxal that is a highly reactive compound disturbing amongst others NO availability (3). In this way a vicious cycle develops leading to enhancement of MVD.
Results:
For our studies we use various techniques to measure microvascular structure and function. To measure both flowmotion and heat-induced hyperemia in skin, we use the Periflux 5000 system.

Obesity:
As stated above, several experimental studies had shown that different pathways in obesity could disturb intracellular signaling in the endothelium, resulting in reduced NO bioavailability. This could attenuate the insulin-mediated glucose disposal in obese subjects. Indeed, previous studies in obese women had shown disturbed microvascular endothelial function during a two hour insulin-clamp. Our aim was to study microvascular function in lean and obese subjects during physiological hyperglycemia (following a meal drink) which leads to a dynamic and transient hyperglycemia and hyperinsulinemia. We studied the skin microcirculation of the wrist using a Periflux 5000 system. Fast Fourier transfer analyses was performed on the flow signal in order to determine the contribution of the five frequency domains to the signal. These five domains represent activity of the endothelium (0.01-0.02 Hz), neurons (0.02-0.06 Hz), myocytes (0.06-0.15 Hz), breathing (0.15-0.40 Hz), and heart rate (0.40-1.60 Hz).
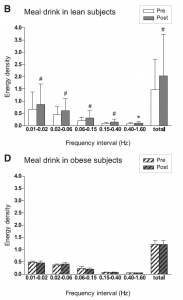
The results are shown in Fig 1. The upper panel shows the flowmotion results in lean individuals before (white bars) and after the meal drink (gray bars). A clear increase in all five domains is visible after the drink. In the lower panel the results of the obese subjects are shown. The meal drink, with subsequent hyperglycemia and hyperinsulinemia, had no effect on microvascular flowmotion. These data clearly demonstrate that in normal physiology, a meal drink leads to stimulation of the microcirculation in lean subjects, possibly via insulin-induced endothelial stimulation. In obese subjects, however, there is no stimulation whatsoever of the microcirculation following a meal drink (4).
Fig 1. (adapted from ref.4): Microvascular flowmotion before and after the mixed-meal drink in lean (B) and obese subjects (D). Bars present means + SD. #P<0.01, *P<0.05 vs. before intake.
Type 2 Diabetes:
To study MVD in pre-diabetes and type 2 diabetes we use data from the Maastricht Study. The Maastricht Study is an observational prospective population-based cohort study that focusses on the etiology of T2DM, its classic complications, and its emerging comorbidities (http://www.demaastrichtstudie.nl/research). This study is unique in its very extensive phenotyping of all participants. One of these phenotyping categories is the microcirculation.
Data from this study has given us the opportunity to get insight into many features of microvascular structure and function in the normal population and in type 2 diabetes in particular. One of the results is that we were able to describe the most important determinants of skin microvascular function in the general population using both flowmotion analyses (5) and heat-induced hyperemia (6). Second, we have shown that microvascular function (skin heat-induced hyperemia and retinal arteriolar reactivity) is disturbed already in subjects with pre-diabetes and that this is exaggerated in type 2 diabetes (7) (Fig. 2). In subsequent analyses, it turned out that hyperglycemia is the most important determinant of this microvascular dysfunction in (pre)diabetes (8). Third, we found that measures of microvascular dysfunction in skin and retina were associated with late-life depression (9). Finally, we have shown that microvascular endothelial dysfunction and capillary rarefaction are associated with albuminuria (10,11).
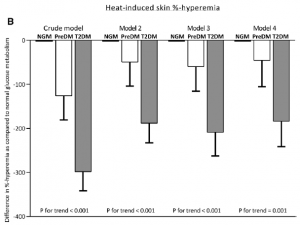
Fig 2. (adapted from ref.7) Multivariable adjusted differences in heat-induced skin %-hyperemia
(B) between individuals with prediabetes (PreDM) and type 2 diabetes (T2DM) compared with normal glucose metabolism (NGM). Bars represent the mean difference with standard error in heat-induced skin %-hyperemia for prediabetes and T2DM as compared with NGM. P values indicate
trend analyses among NGM, prediabetes, and T2DM. NGM is the reference and is set to zero
Dr. Alfons J.H.M. Houben
Dept. of Internal Medicine,
Maastricht University Medical Center+ and School for Cardiovascular Diseases (CARIM),
Maastricht, The Netherlands.
References:
- Houben AJ, Martens RJ, Stehouwer CD. Assessing Microvascular Function in Humans from a Chronic Disease Perspective. J Am Soc Nephrol. 2017 Dec;28(12):3461-3472.
- Jonk AM, Houben AJ, de Jongh RT, et al. Microvascular dysfunction in obesity: a potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Physiology (Bethesda). 2007 Aug;22:252-60.
- Schalkwijk CG. Vascular AGE-ing by methylglyoxal: the past, the present and the future. Diabetologia. 2015 Aug;58(8):1715-9
- Jonk AM, Houben AJ, Schaper NC, et al. Meal-related increases in microvascular vasomotion are impaired in obese individuals: a potential mechanism in the pathogenesis of obesity-related insulin resistance. Diabetes Care. 2011 May;34 Suppl 2:S342-8.
- Muris DM, Houben AJ, Kroon AA, et al. Age, waist circumference, and blood pressure are associated with skin microvascular flow motion: the Maastricht Study. J Hypertens. 2014 Dec;32(12):2439-49.
- Sörensen BM, Houben AJHM, Berendschot TTJM, et al. Cardiovascular risk factors as determinants of retinal and skin microvascular function: The Maastricht Study. PLoS One. 2017 Oct 27;12(10):e0187324.
- Sörensen BM, Houben AJ, Berendschot TT, et al. Prediabetes and Type 2 Diabetes Are Associated With Generalized Microvascular Dysfunction: The Maastricht Study. Circulation. 2016 Nov 1;134(18):1339-1352.
- Sörensen BM, Houben AJ, Berendschot TT, et al. Hyperglycemia Is the Main Mediator of Prediabetes- and Type 2 Diabetes-Associated Impairment of Microvascular Function: The Maastricht Study. Diabetes Care. 2017 Aug;40(8):e103-e105.
- Houben AJ, van Agtmaal M, Sörensen B, et al. Markers of microvascular dysfunction are associated with depressive symptoms: the Maastricht Study. IVBM, Helsinki 2018, abstract.
- Martens RJ, Henry RM, Houben AJ, et al. Capillary Rarefaction Associates with Albuminuria: The Maastricht Study. J Am Soc Nephrol. 2016 Dec;27(12):3748-3757.
- Martens RJ, Houben AJ, Kooman JP, et al. Microvascular endothelial dysfunction is associated with albuminuria: the Maastricht Study. J Hypertens. 2018 May;36(5):1178-1187.
References:
- Binggeli C. et al. Statins enhance postischemic hyperemia in the skin circulation of hypercholesterolemic patients. J Amer Collage of Card 2003 42; 1:71-77
- Ruano J. et al. Phenolic content of virgin olive oil improves ischemic reactive hyperemia in hypercholesterolemic patients. J Amer Collage of Card 2005 46; 10:1864-1868
- Roustit M. et al, Excellent reproducibility of laser speckle contrast imaging to assess skin microvascular reactivity Microvascular Research, 2010
- Ann Humeau-Heurtier et al. Excellent inter- and intra-observer reproducibility of microvascular tests using laser speckle contrast imaging, Clinical Hemorheology and Microcirculation, 2013
- Roustit et al, Assessment of endothelial and neurovascular function in human skin microcirculation, Trends in Pharmacological Sciences, 2013, 34(7):373-84
- Kellogg D.L. Jr. In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. J Appl Physiol 2006;100:1709-1718.
- Gooding K.M. et al, Maximum Skin Hyperaemia Induced by Local Heating: Possible Mechanism. J Vasc Res 2006;43:270-277
- Minson C.T. et al, Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 2001 91:1619-1626
- Minson C.T. et al, Thermal provocation to evaluate microvascular reactivity in human skin. J Appl Physiol 2010 109:1239-1246
- Blaauw J. et al. Abnormal endothelium-dependent microvascular reactivity in recently preeclamptic women. Amer Coll of Obstetricians and Gynecologists, 2005, 105; 3:626-632
- Durand S. et al. Prostaglandins participate in the late phase of the vascular response to acetylcholine iontophoresis in humans. J Physiol, 2004, 811-819
Disclaimer: It is possible that the products on the Perimed website may not be cleared for sale in all markets.